Is H2 Polar Or Nonpolar
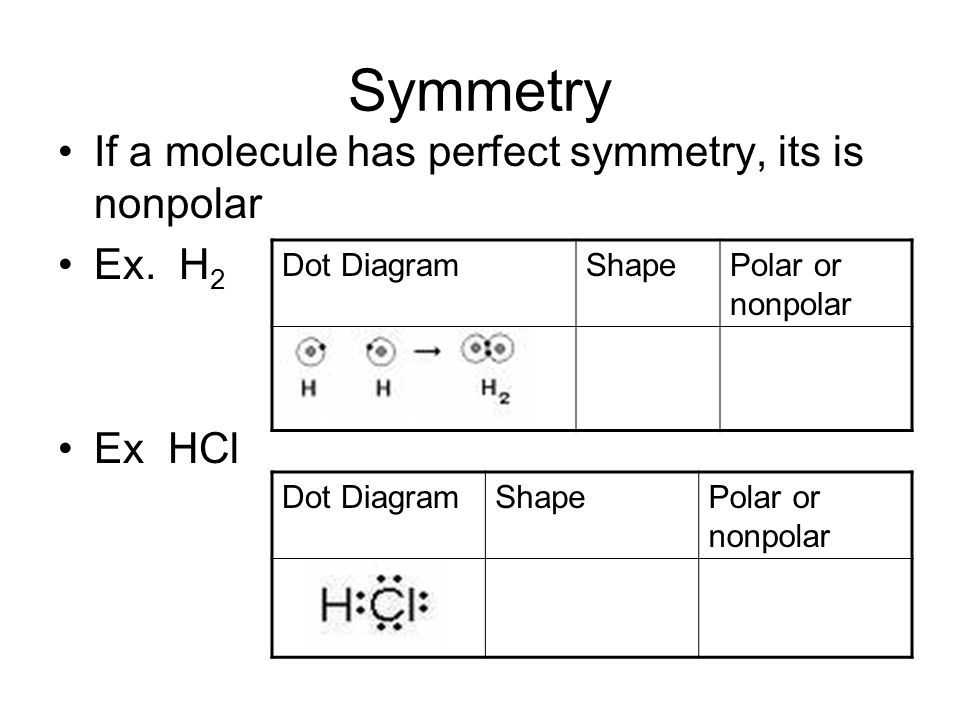
H2 is a covalent nonpolar covalent molecule because when one nonmetal combines with another nonmetal it usually forms a covalent molecule or compound. Other non polar gases are oxygen nitrogen ethylene and carbon dioxide gas.
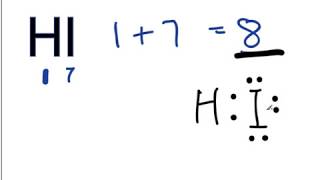
Is Hi Polar Or Nonpolar
Is H2 polar or nonpolar.
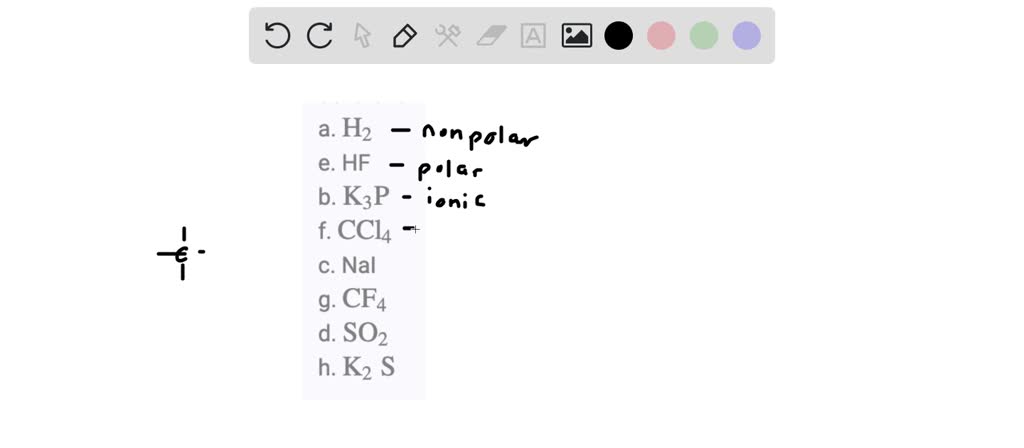
. The Molecules that are made of the same element are generally seen nonpolar. Molecules that have symmetry in its stru See more. For nonpolar molecules check if.
Some examples can be CH4 C2H6 3. A nonpolar covalent bond is when two atoms share electrons equally. Learn to determine if H2 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and look and.
Similarly which one is polar H2 or h2o. In nonpolar molecules there is no occurrence of partial positive and negative charge on the atoms because of the same electronegativity different between the atoms. If the electronegativity difference ΔEN is.
Molecules containing only carbon and hydrogen atoms ie. Some examples can be N2 F2 H2 He Ne Ar Xe. In order for the bond to be polar there has to be difference between the electronegativity of the atoms.
And we also have to check the molecular geometry of H2O. Hydrogen gas H2 is nonpolar because both hydrogen atoms have the same electronegativity so the difference in electronegativity is 0 which means the bond is nonpolar. These covalent bonds are non-polar.
Polar bonds are formed when the two atoms involved in the bond have a large difference in their electronegativity values. H2 or Hydrogen gas is a NONPOLAR molecule because any two bonding atoms whose electronegativity difference value is less than 04 forms a nonpolar bond. H2 is a nonpolar molecule because of the linear geometrical structure and the same electronegativity of both hydrogen.
Added an answer on March 9 2022 at 1130 pm. Either an equal sharing of electrons or an unequal sharing of electrons occurs. Have a look at the above image.
Answer 1 of 3. The gas hydrogen is a non polar element. The electronegativity of both atoms in the H-H bond is identical.
Now in the next step we have to check whether these O-H bonds are polar or nonpolar. If the electronegativity difference ΔEN is less than 04 then the bond is nonpolar covalent bond. As the electronegativity difference between Se and H is less than 040 therefore each Se-H covalent bond in H2Se H 2 S e is nonpolar.
Is H2 SE polar or nonpolar. So Is H2 polar or nonpolar. Water H 2 O is polar because of the bent shape of the molecule.
The answer is dHF. The electronegativity value of hydrogen is. This is the most stable kind of bond.
Two atoms that share their electrons equally form a Nonpolar bond while two bonded atoms that share electrons.
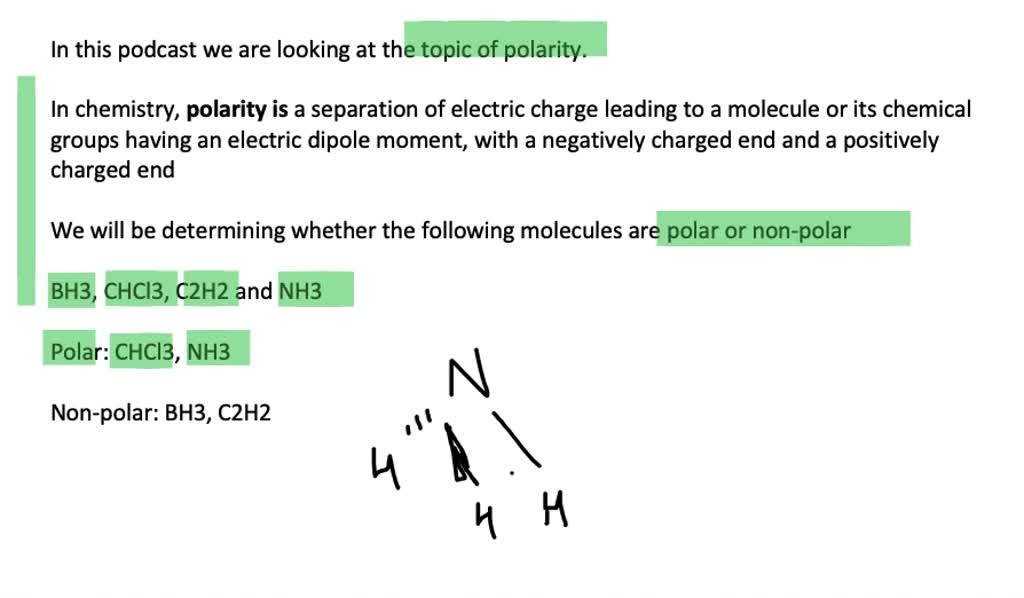
Solved Classify Each Molecule As Polar Or Nonpolar A Bh3 B Chcl3 C C2 H2 D Nh3
Polar Vs Nonpolar Molecules Ppt Video Online Download
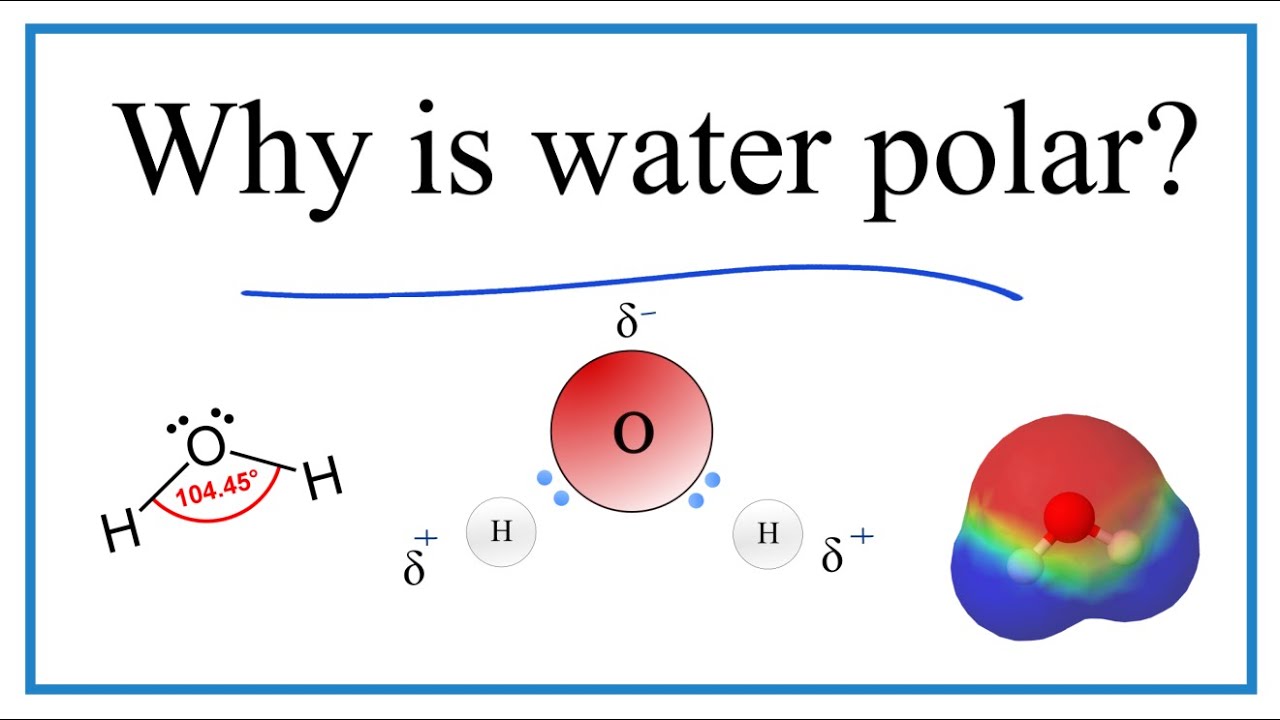
Why Is Water H2o A Polar Molecule Youtube
Why Is H2 A Non Polar Molecule Quora

Is H2 Polar Or Nonpolar Techiescientist

Which Molecule Contains Both Polar And Nonpolar Covalent Bond
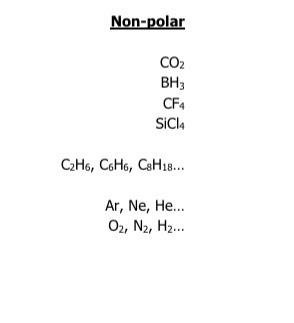
Solved What Category Of Substances Is In This List Check All Chegg Com

Types Of Covalent Bonds Polar And Nonpolar Manoa Hawaii Edu Exploringourfluidearth
Hydrogen Molar Mass What S Insight
Solved Arrange The Following Molecules From Most To Least Polar And Explain Your Order Ch 4 Cf
Why Is H2 A Non Polar Molecule Quora
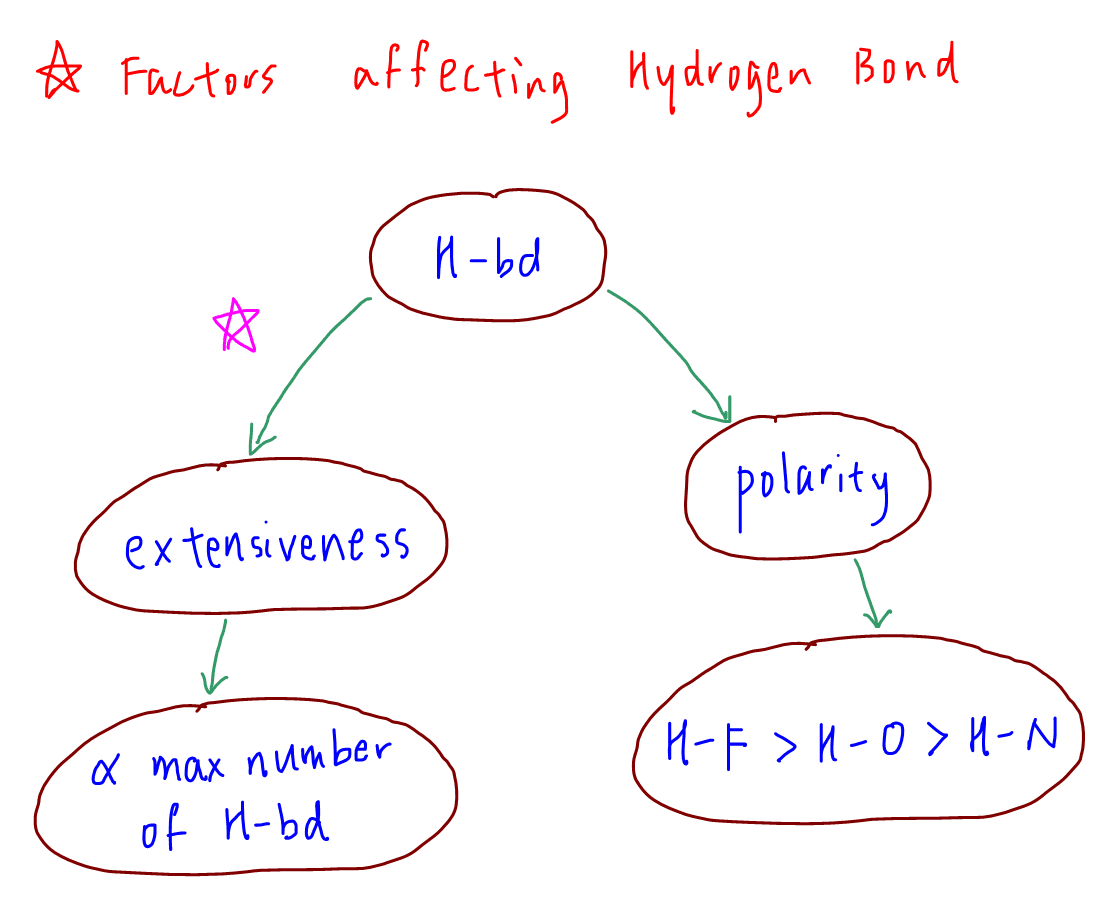
Factors Affecting Hydrogen Bond
Solved Molecular Geometry Name And Sketch Polar Or Chegg Com

Media Portfolio

Identify The Non Polar Molecule In The Set Of Compounds Given Hcl Hf H2 Hbr
Why Is H2 Nonpolar Molecule While Hf Is A Polar Molecule Quora

Hf Is Polar Or Nonpolar Covalent Bond Covalent Bonding Polar Molecules